Master’s Thesis:
From parthanatos to PAANIB-1:
A multimedia exploration of cell death mechanisms and therapeutic strategies for Parkinson’s Disease
objectives:
- Craft an engaging 3D animation that improves public understanding of the Parthanatos pathway and its relationship to the cGAS-STING pathway in PD
- Raise awareness of PAANIB-1, a previously unvisualized first-in-class MIF-inhibitor, as a novel potential therapeutic target for PD
- Design an interactive web module for graduate students that expands on the molecular mechanisms of PD pathogenesis and interventional strategies
View the interactive web module below:
How does the brain deteriorate in parkinson's disease?


What are the mechanisms of the Parthanatos cell death pathway?
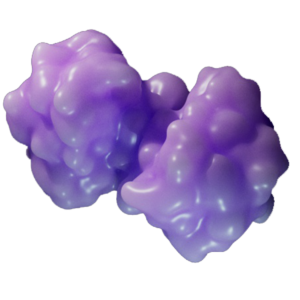
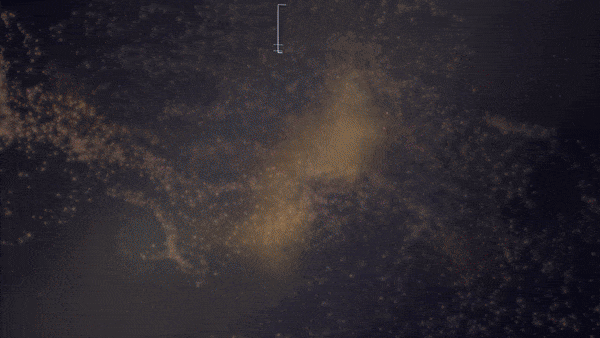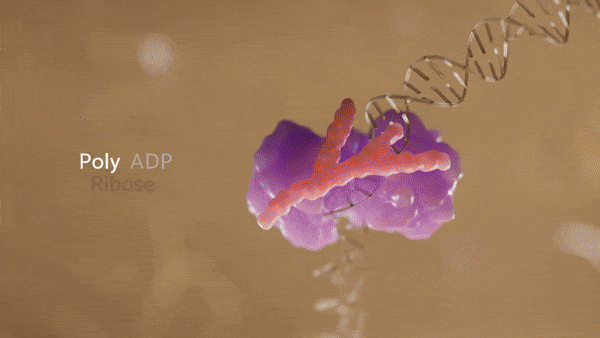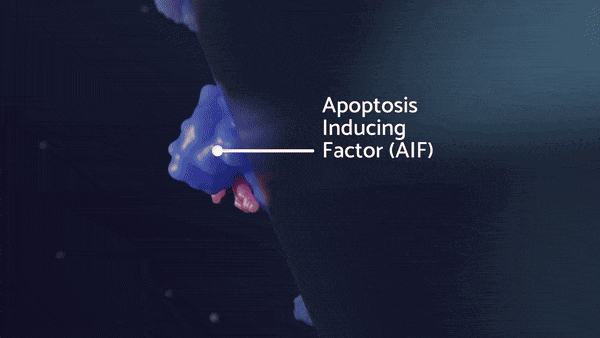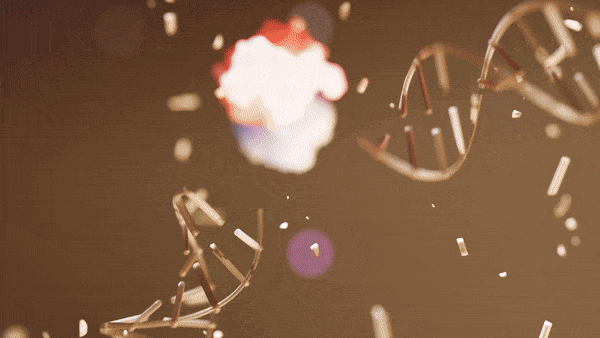
Poly ADP-ribose polymerase–1 (PARP–1) is a nuclear protein that is activated by binding to DNA lesions. It catalyzes poly ADP-ribosylation of nuclear acceptor proteins, including PARP-1 itself, to recruit DNA repair machinery to damaged sites. Excessive DNA damage causes PARP-1 to overproduce PAR, triggering the parthanatos pathway.
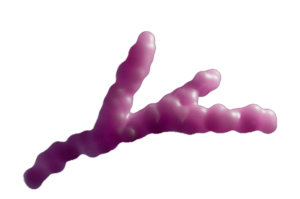
Poly ADP-ribose (PAR) is a polymer generated by PARP1 enzyme in response to DNA damage. Composed of negatively charged ADP-ribose units, it forms chains on target proteins, aiding in DNA repair by recruiting repair factors. PAR acts as a signaling platform, orchestrating the repair process at damaged sites.
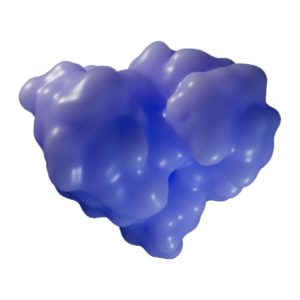
Apoptosis-Inducing Factor (AIF), primarily localized within the outer membrane of the mitochondria, normally regulates cellular metabolism and chromatin condensation for apoptosis. AIF can interact with PAR and influence AIF’s translocation to the nucleus upon heightened cellular stress.
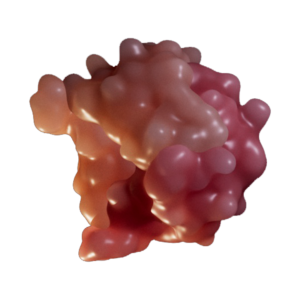
Macrophage Migration Inhibitory Factor (MIF) normally regulates cytokine production and inhibits macrophage migration. MIF is the major nuclease involved in large-scale DNA fragmentation during parthanatos, and can also exacerbate PAR formation. AIF may enhance MIF nuclease activity by increasing its binding to ssDNAs.

During parthanatos, MIF is carried into the nucleus by AIF, where MIF nuclease preferentially binds to stem-loop single-stranded DNA and cleaves 3′ unpaired bases of stem-loop ssDNA into 20‐ to 50‐kb fragments. The cGAS-STING pathway can respond to this self-DNA that mislocalizes to the cytoplasm and drives autoinflammation.
PAANIB-1: A potential therapeutic treatment for Parkinson's
PAANIB-1 is Highly Selective
PAANIB-1 specifically targets the nuclease domain of MIF. This reduces potential side effects arising from altering MIF’s other neuroprotective functions, such as tautomerase, oxidoreductase, and glucocorticoid suppressing activities.
PAANIB-1 is Brain Penetrant
PAANIB-1 efficiently crosses the blood-brain barrier (BBB), achieving potent therapeutic concentrations in the brain following administration.
PAANIB-1 Prevents Symptoms of PD
Across several mouse models, PAANIB-1 offered protection from degeneration of dopamine neurons and the behavioral symptoms associated with PD.
full Thesis animation:
Links and resources
Beitz, Janice M. “Parkinson’s Disease: a Review.” Frontiers in Bioscience S6, no. 1 (2014): 65–74. https://doi.org/10.2741/s415.
Meade, Richard M., David P. Fairlie, and Jody M. Mason. “Alpha-Synuclein Structure and Parkinson’s Disease – Lessons and Emerging Principles.” Molecular Neurodegeneration 14, no. 1 (July 22, 2019). https://doi.org/10.1186/s13024-019-0329-1.
“Parkinson’s Disease: Causes, Symptoms, and Treatments.” National Institute on Aging, U.S. Department of Health and Human Services, 14 Apr. 2022, www.nia.nih.gov/health/parkinsons-disease#causes.
Galluzzi, Lorenzo, Ilio Vitale, Stuart A. Aaronson, John M. Abrams, Dieter Adam, Patrizia Agostinis, Emad S. Alnemri, et al. “Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018.” Cell Death & Differentiation 25, no. 3 (January 23, 2018): 486–541. https://doi.org/10.1038/s41418-017-0012-4.
Park, Hyejin, Tae-In Kam, Ted M. Dawson, et al. “Poly (ADP-ribose) (par)-dependent cell death in neurodegenerative diseases.” Cell Death Regulation In Health And Disease – Part C, 2020, pp. 1–29, https://doi.org/10.1016/bs.ircmb.2019.12.009.
Huang, Ping, Guangwei Chen, Weifeng Jin, Kunjun Mao, Haitong Wan, and Yu He. “Molecular Mechanisms of Parthanatos and Its Role in Diverse Diseases.” International Journal of Molecular Sciences 23, no. 13 (June 30, 2022): 7292. https://doi.org/10.3390/ijms23137292.
Wang, Yingfei, Ran An, George K. Umanah, Hyejin Park, Kalyani Nambiar, Stephen M. Eacker, BongWoo Kim, et al. “A Nuclease That Mediates Cell Death Induced by DNA Damage and Poly(Adp-Ribose) Polymerase-1.” Science 354, no. 6308 (October 7, 2016). https://doi.org/10.1126/science.aad6872.
Bai, Peter. “Biology of Poly(Adp-Ribose) Polymerases: The Factotums of Cell Maintenance.” Molecular Cell 58, no. 6 (June 2015): 947–58. https://doi.org/10.1016/j.molcel.2015.01.034.
Hinkle, Jared T., et al. “Sting mediates neurodegeneration and neuroinflammation in nigrostriatal α-synucleinopathy.” Proceedings of the National Academy of Sciences, vol. 119, no. 15, 8 Apr. 2022, https://doi.org/10.1073/pnas.2118819119.
Li, Tuo, and Zhijian J. Chen. “The CGAS–Cgamp–Sting Pathway Connects DNA Damage to Inflammation, Senescence, and Cancer.” Journal of Experimental Medicine 215, no. 5 (April 5, 2018): 1287–99. https://doi.org/10.1084/jem.20180139.
De Virgilio, Armando, Antonio Greco, Giovanni Fabbrini, Maurizio Inghilleri, Maria Ida Rizzo, Andrea Gallo, Michela Conte, Chiara Rosato, Mario Ciniglio Appiani, and Marco de Vincentiis. “Parkinson’s Disease: Autoimmunity and Neuroinflammation.” Autoimmunity Reviews 15, no. 10 (August 4, 2016): 1005–11. https://doi.org/10.1016/j.autrev.2016.07.022.
Chen, Qi, Lijun Sun, and Zhijian J. Chen. “Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing.” Nature immunology 17, no. 10 (2016): 1142-1149.
Motwani, Mona, Scott Pesiridis, and Katherine A. Fitzgerald. “DNA sensing by the cGAS–STING pathway in health and disease.” Nature Reviews Genetics 20, no. 11 (2019): 657-674.
Decout, Alexiane, Jason D. Katz, Shankar Venkatraman, and Andrea Ablasser. “The cGAS–STING pathway as a therapeutic target in inflammatory diseases.” Nature Reviews Immunology 21, no. 9 (2021): 548-569.
Berger, Nathan A., et al. “Opportunities for the Repurposing of PARP Inhibitors for the Therapy of Non‐oncological Diseases.” British Journal of Pharmacology 175, no. 2 (March 26, 2017): 192–222. https://doi.org/10.1111/bph.13748.
Biswas, Devanik, et al. “Pharmacologic inhibition of MIF nuclease: A new treatment paradigm to treat cell death.” Clinical and Translational Medicine, vol. 12, no. 9, 23 Aug. 2022, https://doi.org/10.1002/ctm2.1044.
Park, Hyejin, et al. “Paan/MIF nuclease inhibition prevents neurodegeneration in parkinson’s disease.” Cell, vol. 185, no. 11, 26 May 2022, https://doi.org/10.1016/j.cell.2022.04.020.
Patel, Jaimin, Valina L. Dawson, and Ted M. Dawson. “Blocking the Self‐destruct Program of Dopamine Neurons through Macrophage Migration Inhibitory Factor Nuclease Inhibition.” Movement Disorders, February 23, 2024. https://doi.org/10.1002/mds.29748.

